Bảng tuần hoàn hóa học tiếng Anh là gì?
Bảng tuần hoàn hóa học tiếng Anh được gọi là "Periodic Table of Elements."
Bảng tuần hoàn hóa học là một bảng sắp xếp các nguyên tố hóa học theo thứ tự tăng dần của số nguyên tử. Nó được sử dụng để tổ chức, phân loại và hiển thị thông tin về các nguyên tố hóa học, bao gồm tên, ký hiệu hóa học, số nguyên tử, khối lượng nguyên tử, cấu trúc điện tử và tính chất hóa học của chúng.
Hiện tại, bảng tuần hoàn hóa học chứa 118 nguyên tố hóa học. Tuy nhiên, hãy lưu ý rằng số lượng nguyên tố có thể thay đổi theo thời gian do khám phá và nghiên cứu mới.
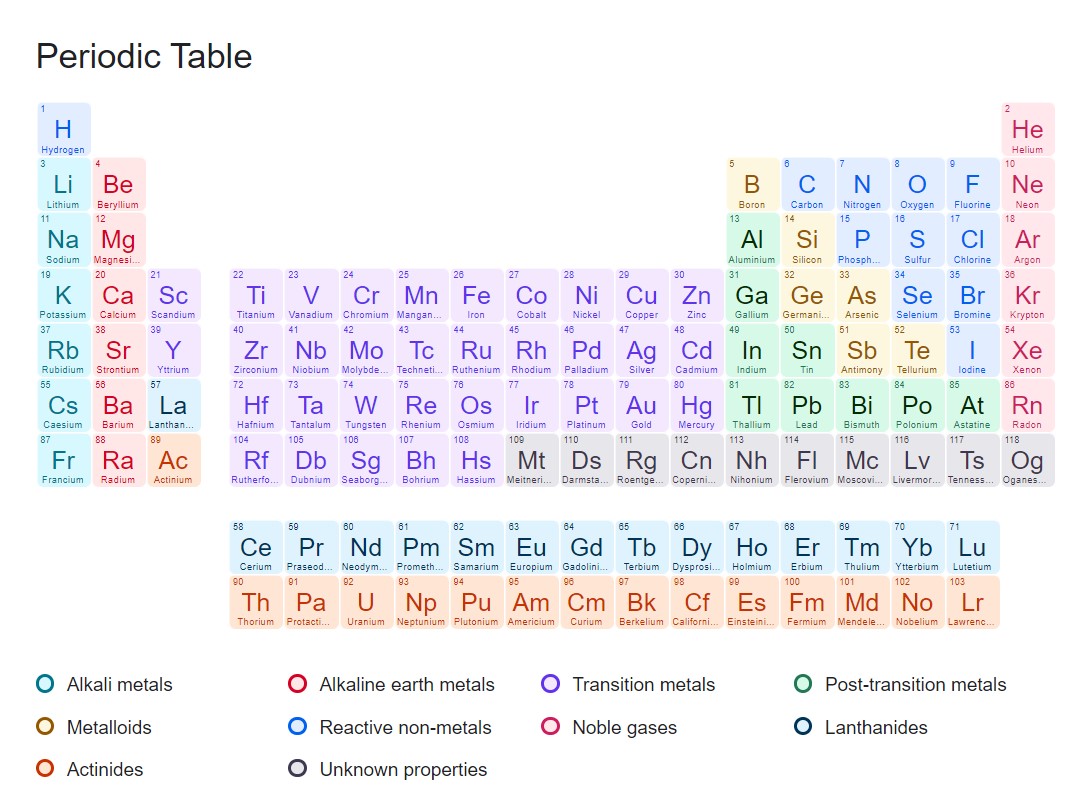 Bảng tuần hoàn hóa học tiếng Anh
Bảng tuần hoàn hóa học tiếng Anh
Bảng tổng hợp đầy đủ tên tiếng Anh của các nguyên tố hóa học
STT
Tên nguyên tố
Tên Tiếng Việt
Kí hiệu
Cách phát âm
1
Hydrogen
Hiđrô
H
/ˈhaɪ.drə.dʒən/
2
Helium
Heli
He
/ˈhiː.li.əm/
3
Lithium
Liti
Li
/ˈlɪθ.i.əm/
4
Beryllium
Berili
Be
/bəˈrɪl.i.əm/
5
Boron
Bari
B
/ˈbɔːrɒn/
6
Carbon
Cacbon
C
/ˈkɑːr.bən/
7
Nitrogen
Nitơ
N
/ˈnaɪ.trə.dʒən/
8
Oxygen
Ôxy
O
/ˈɒk.sɪ.dʒən/
9
Fluorine
Flo
F
/ˈflʊər.iːn/
10
Neon
Neon
Ne
/ˈniː.ɒn/
11
Sodium
Natri
Na
/ˈsəʊ.di.əm/
12
Magnesium
Magiê
Mg
/mæɡˈniːziəm/
13
Aluminum
Nhôm
Al
/əˈluː.mɪ.ni.əm/
14
Silicon
Silic
Si
/ˈsɪl.ɪ.kən/
15
Phosphorus
Photpho
P
/ˈfɒs.fər.əs/
16
Sulfur
Lưu huỳnh
S
/ˈsʌl.fər/
17
Chlorine
Clorin
Cl
/ˈklɔːr.iːn/
18
Argon
A-go-ni
Ar
/ˈɑːɡɒn/
19
Potassium
Kali
K
/pəˈtæs.i.əm/
20
Calcium
Canxi
Ca
/ˈkæl.si.əm/
21
Scandium
Scanđi
Sc
/ˈskæn.di.əm/
22
Titanium
Titan
Ti
/tɪˈteɪ.ni.əm/
23
Vanadium
Vanađi
V
/vəˈneɪ.di.əm/
24
Chromium
Crôm
Cr
/ˈkroʊ.mi.əm/
25
Manganese
Mangan
Mn
/ˈmæŋ.ɡəniz/
26
Iron
Sắt
Fe
/ˈaɪ.ərn/
27
Cobalt
Coba
Co
/ˈkoʊ.bɒlt/
28
Nickel
Niken
Ni
/ˈnɪk.əl/
29
Copper
Đồng
Cu
/ˈkɑː.pɚ/
30
Zinc
Kẽm
Zn
/zɪŋk/
31
Gallium
Galli
Ga
/ˈɡæl.i.əm/
32
Germanium
Gecmani
Ge
/ˈdʒɜːr.meɪ.ni.əm/
33
Arsenic
Asen
As
/ˈɑːr.sə.nɪk/
34
Selenium
Selen
Se
/sɪˈliː.ni.əm/
35
Bromine
Brom
Br
/ˈbroʊ.miːn/
36
Krypton
Kripton
Kr
/ˈkrɪp.tɒn/
37
Rubidium
Rubiđi
Rb
/ˈruː.bi.di.əm/
38
Strontium
Srotni
Sr
/ˈstrɒn.ti.əm/
39
Yttrium
Ytri
Y
/ˈɪtri.əm/
40
Zirconium
Zicroni
Zr
/zɜːrˈkoʊ.ni.əm/
41
Niobium
Niobi
Nb
/ˈnaɪ.oʊ.bi.əm/
42
Molybdenum
Molipđen
Mo
/məˈlɪb.də.nəm/
43
Technetium
Teken
Tc
/tɛkˈniː.ʃi.əm/
44
Ruthenium
Ruteni
Ru
/ruːˈθiː.ni.əm/
45
Rhodium
Rôdi
Rh
/ˈroʊ.di.əm/
46
Palladium
Paladi
Pd
/pəˈleɪ.di.əm/
47
Silver
Bạc
Ag
/ˈsɪl.vər/
48
Cadmium
Cadimi
Cd
/ˈkæd.mi.əm/
49
Indium
Inđi
In
/ˈɪn.di.əm/
50
Tin
Thiếc
Sn
/tɪn/
51
Antimony
Antimon
Sb
/ˈæn.təˌmoʊ.ni/
52
Tellurium
Tellu
Te
/tɛˈlʊər.i.əm/
53
Iodine
Iot
I
/ˈaɪ.əˌdiːn/
54
Xenon
Xênon
Xe
/ˈziː.nɒn/
55
Cesium
Xesi
Cs
/ˈsiːziəm/
56
Barium
Bari
Ba
/ˈbɛəriəm/
57
Lanthanum
Lantan
La
/ˈlæn.θə.nəm/
58
Cerium
Xeri
Ce
/ˈsɪəriəm/
59
Praseodymium
Praseodi
Pr
/ˌpreɪz.iˈoʊ.di.mi.əm/
60
Neodymium
Neođim
Nd
/ˌniː.oʊˈdɪ.mi.əm/
61
Promethium
Promeđi
Pm
/prəˈmiːθiəm/
62
Samarium
Samari
Sm
/səˈmɛəriəm/
63
Europium
U-rô-pi
Eu
/jʊˈroʊpiəm/
64
Gadolinium
Gado-lin
Gd
/ˌɡædəˈlɪniəm/
65
Terbium
Terbi
Tb
/ˈtɜrbiəm/
66
Dysprosium
Diprosi
Dy
/dɪˈsprɒziəm/
67
Holmium
Holmi
Ho
/ˈhoʊlmiəm/
68
Erbium
Eri
Er
/ˈɜrbiəm/
69
Thulium
Thu-li
Tm
/ˈθjuːliəm/
70
Ytterbium
Ytterbi
Yb
/ˈɪtərbiəm/
71
Lutetium
Lu-tê-xi
Lu
/luːˈtiːʃiəm/
72
Hafnium
Hafni
Hf
/ˈhæfniəm/
73
Tantalum
Tan-ta-lum
Ta
/ˈtæntələm/
74
Tungsten
Tung-xten
W
/ˈtʌŋstən/
75
Rhenium
Re-ni
Re
/ˈriːniəm/
76
Osmium
O-xi-um
Os
/ˈɒzmiəm/
77
Iridium
I-ri-đi-um
Ir
/ɪˈrɪdiəm/
78
Platinum
Ba-chi
Pt
/ˈplætɪnəm/
79
Gold
Vàng
Au
/ɡoʊld/
0
Mercury
Thuỷ ngân
Hg
/ˈmɜːrkjʊri/
81
Thallium
Talium
Tl
/ˈθæliəm/
82
Lead
Chì
Pb
/lɛd/
83
Bismuth
Bizmut
Bi
/ˈbɪzməθ/
84
Polonium
Poloni
Po
/pəˈloʊniəm/
85
Astatine
Astatin
At
/ˈæstətiːn/
86
Radon
Radon
Rn
/ˈreɪdɒn/
87
Francium
Franxi
Fr
/ˈfrænsiəm/
88
Radium
Radium
Ra
/ˈreɪdiəm/
89
Actinium
Actini
Ac
/ækˈtɪniəm/
90
Thorium
Tori
Th
/ˈθɔːriəm/
91
Protactinium
Pro-tac-ti-ni
Pa
/ˌproʊtækˈtɪniəm/
92
Uranium
U-ran
U
/jʊˈreɪniəm/
93
Neptunium
Nêp-tun
Np
/nɛpˈtjuːniəm/
94
Plutonium
Plu-toni
Pu
/pluːˈtoʊniəm/
95
Americium
A-me-ri-xi
Am
/ˌæməˈrɪsiəm/
96
Curium
Cu-ri-um
Cm
/ˈkjʊəriəm/
97
Berkelium
Ber-ke-li-um
Bk
/ˈbɜːrkliəm/
98
Californium
Cali-pho-ni
Cf
/ˌkælɪˈfɔːrniəm/
99
Einsteinium
A-in-x-tei-ni
Es
/aɪnˈstaɪniəm/
100
Fermium
Fê-mi
Fm
/ˈfɜːrmiəm/
101
Mendelevium
Menđelevi
Md
/ˌmɛndəˈliːviəm/
102
Nobelium
Nobelium
No
/noʊˈbiːliəm/
103
Lawrencium
Lawrenxi
Lr
/lɔːˈrɛnsiəm/
104
Rutherfordium
Rutherfordi
Rf
/ˌrʌðərˈfɔːrdiəm/
105
Dubnium
Đubni
Db
/ˈduːbniəm/
106
Seaborgium
Si-bor-gi
Sg
/ˈsiːbɔːrɡiəm/
107
Bohrium
Bo-ri
Bh
/ˈboʊriəm/
108
Hassium
Ha-xi
Hs
/ˈhæsiəm/
109
Meitnerium
Meitneri
Mt
/maɪtˈnɪəriəm/
110
Darmstadtium
Darmstadi
Ds
/dɑːrmˈʃtɑːtiəm/
111
Roentgenium
Rontgeni
Rg
/ˈrɛntɡəniəm/
112
Copernicium
Copernici
Cn
/ˌkoʊpərˈnɪsiəm/
11
Nihonium
Nihoni
Nh
/ˈniːhoʊniəm/
114
Flerovium
Flerovi
Fl
/flɛˈroʊviəm/
115
Moscovium
Moscovium
Mc
/ˈmɒskoʊviəm/
116
Livermorium
Livermorium
Lv
/ˌlɪvərˈmɔːriəm/
117
Tennessine
Tennessin
Ts
/tɛˈnɛsiːn/
118
Oganesson
Oganesson
Og
/ˈoʊɡənɛsən/
Tham khảo: Từ vựng tiếng Anh chuyên ngành hóa học và bài tập vận dụng cơ bản
Bài đọc ứng dụng - Chủ đề “Tên tiếng Anh của các nguyên tố hóa học”
The Histories Hidden in the Periodic Table
From poisoned monks and nuclear bombs to the “transfermium wars,” mapping the atomic world hasn’t been easy.
The story of the fifteenth element began in Hamburg, in 1669. The unsuccessful glassblower and alchemist Hennig Brandt was trying to find the philosopher’s stone, a mythical substance that could turn base metals into gold. Instead, he distilled something new. It was foamy and, depending on the preparation, yellow or black. He called it “cold fire,” because it glowed in the dark. Interested parties took a look; some felt that they were in the presence of a miracle. “If anyone had rubbed himself all over with it,” one observer noted, “his whole figure would have shone, as once did that of Moses when he came down from Mt. Sinai.” Robert Boyle, the father of modern chemistry, put some on his hand and noted how “mild and innocent” it seemed. Another scientist saw particles in it twinkling “like little stars.”
At first, no one could figure out what the Prometheus of Hamburg had stolen. After one of Brandt’s confidants provided a hint—the main ingredient was “somewhat that belonged to the Body of Man”—Boyle deduced that he and his peers had been smearing themselves with processed urine. As the Cambridge chemist Peter Wothers explains in his new history of the elements, “Antimony, Gold, and Jupiter’s Wolf” (Oxford), Brandt’s recipe called for a ton of urine. It was left out in buckets long enough to attract maggots, then distilled in hot furnaces, creating a hundred and twenty grams of “cold fire.” Brandt believed that, if he could collect enough of this substance, he might be able to create the philosopher’s stone. In 1678, the Duke of Saxony asked him to collect a hundred tons of urine from a garrison of soldiers and render it into what Boyle and others soon started to call phosphorus—Latin for “light-bearer.”
The soapy phosphorus that Brandt cooked up was a curiosity. But, in England, Boyle began producing it in a purer, more solid form, which turned out to be highly flammable. Another scientist toying with Boyle’s phosphorus found that “if the Privy Parts be therewith rubbed, they will be inflamed and burning for a good while after.” Boyle, for his part, wondered whether it could be harnessed as a starter for gunpowder. (His assistant, the apothecary Ambrose Godfrey, set his head on fire and burned “two or three great holes in his breeches” while investigating the substance.) The phosphorus industry grew throughout the eighteenth century, in part because physicians wrongly believed that it had medicinal value. In the eighteen-hundreds, match producers found that wood splints tipped with phosphorus were less dangerous than their sulfur-coated predecessors; not long afterward, the discovery that electric furnaces could extract phosphorus from ore at a large scale led to the development of explosives. In the Second World War, in what Wothers calls “a tragic twist of fate,” Hamburg, Brandt’s hometown, was destroyed by Allied bombers dropping phosphorus munitions.
Wothers finds many such twists in the stories hidden behind the squares of the periodic table. Antimony (element No. 51) is a lustrous mineral; four thousand years ago, people carved vases out of it, and it appears in cosmetic regimes described in the Old Testament. According to an account given by the seventeenth-century apothecary and alchemist Pierre Pomet (offered up by Wothers as possibly apocryphal), antimony got its name from the story of a German monk who fed it to his fellow brethren. The monk had given some to a few pigs, who vomited at first but then grew healthy and fat. Unfortunately, every monk who ingested it died. “This therefore was the reason of this Mineral being called Antimony,” Pomet wrote, “as being destructive of the Monks.” (In a less fatal episode, a nineteenth-century doctor and his friends consumed fifteen milligrams of tellurium each: they had garlic breath for eight months.)
The names of the elements have long been a source of contention and incomprehension. Hydrogen, Wothers points out, is Greek for “water-former,” while oxygen is Greek for “acid-former”; in fact, it’s hydrogen that bonds together with other elements to make acids and oxygen that bonds hydrogen to make water. “Aluminium,” Charles Dickens wrote, in 1856, is “a fossilized part of Latin speech, about as suited to the mouths of the populace as an ichthyosaurus cutlet or a dinornis marrow-bone.” (It has its root in the Latin for “bitter salt,” after the clay from which the once-precious metal was derived; Dickens’s suggestions—“loam-silver” and “glebe-gold”—weren’t much better.) The French chemist Marguerite Perey, a protégée of Marie Curie, discovered an element of her own, in 1939. She wanted to call it “catium,” to honor the particle’s strong attraction to cathodes, devices used to send electricity through a chemical substance; Curie’s daughter, Irène Joliot-Curie, worried that English speakers would associate the element with house cats. Perey, being French, decided to call it francium instead.
Many historians date the invention of the periodic table to the publication, a hundred and fifty years ago, of a textbook by the Russian chemist Dmitri I. Mendeleev. But Eric Scerri, the author of “The Periodic Table: Its Story and Its Significance” (Oxford) and a philosopher of chemistry at U.C.L.A.—he studies the history of questions such as “What is an element, really?”—bristles at the notion that Mendeleev revolutionized science when he brought chemical periodicity into clear relief. Periodicity—the idea that larger atoms chime with smaller atoms in a regular way, like notes on a keyboard—didn’t emerge as a bolt from the blue, Scerri argues. It came into focus through the work of a host of scientists; as it did so, ideas that by then were long disdained, such as alchemy, turned out to be right in some respects, and essentially wrong ideas, such as the irreducibility of the elements, turned out to be productive ways of thinking, anyway. Some of the eighteenth- and nineteenth-century chemists who began to notice patterns among certain elements were actually retracing the paths of ancient Greek atomists such as Democritus and Leucippus, who, in the fifth century B.C., had argued that invisible and indivisible particles made up everything we could see and touch. The atomists believed that those particles were myriad in shape and size, and that their perceptible properties came from the structures they formed when they hooked together.
By the Middle Ages, atomistic ideas had been mostly eclipsed by Aristotle’s theory that four principal elements—fire, earth, water, and air—combined to form the various objects in the universe. But atomism never went away completely. Renaissance scholars believed in a wide variety of elemental schemes. Wothers’s book reprints some of the diagrams that mixed these ideas in advance of the periodic table: a seventeenth-century engraving of the “seven metals” shows seven Roman gods brandishing ancient chemical symbols (the deities reminded viewers that iron was from Mars and copper from Venus); another shows the seven metals and Aristotle’s four elements in a triangular arrangement. Ringing the whole diagram is a Latin motto: “Although I am invisible, I am nonetheless the father and mother of all visible earthly bodies.”
You didn’t have to be a scholar, of course, to believe in a world made up of more than four elements. Seventeenth-century miners, Wothers writes, distinguished between different kinds of air: they called the lighter air that swirled at the top of caves “fire-damp,” because it easily burst into flames, and the heavy clouds that hung near the ground “choke-damp,” because they made it hard to breathe. In the eighteenth century, locals dubbed a cave near Naples the Grotta del Cane: dogs who wandered into the cave, unable to raise their heads above the gas seeping out of the Earth, soon began to choke to death; once returned to the open air, the animals would revive.
As these observations proliferated, so did the conviction that there must be many different elements. By the end of the eighteenth century, scientists, combining substances, began realizing that certain materials always reacted in the same proportions, which suggested that they had different underlying masses. (It always seemed to take a little more ammonia than it did magnesia to neutralize the same amount of sulfuric acid.) In 1803, the English scientist John Dalton proposed that atoms were at work in such reactions; he encouraged his peers to help him determine how much these invisible entities weighed. What Scerri calls a “craze for searching for numerical regularities” began. Chemists soon noticed patterns when they grouped elements into sets of three by atomic weight. (Lithium, sodium, and potassium, for example, all fizz or explode in water; it turned out that sodium’s atomic weight is the average of lithium’s and potassium’s.) Such experiments offered glimpses of an order within the elemental universe. But the work was frustrating. In 1836, the chemist Jean Baptiste André Dumas, a disciple of Dalton, threw up his hands in despair. “What remains of the ambitious excursion we allowed ourselves into the domain of atoms?” he wrote. “If I were master I would erase the word ‘atom’ from the science.”
Other chemists pressed on. As atomic weights grew more accurate, more patterns emerged. In 1864, the German chemist Julius Lothar Meyer published a table of twenty-eight elements. Meyer’s elements, arranged mostly by increasing weight, were also lined up according to their common chemical properties, which repeated at regular intervals. Five years later, Mendeleev published his own periodic table, which steadily evolved into the version we use today. Like Meyer, Mendeleev had organized his particles into a rough grid, its rows containing elements with similar properties. But he also garnished his table with many tempting question marks and empty spaces, and made explicit elemental prophecies. Mendeleev accurately predicted the existence of then-undiscovered elements, such as gallium and germanium, and foretold their interactions with other elements.Mendeleev’s predictions were wrong as often as they were right. But, Scerri explains, the Russian chemist was a master storyteller and, compared to Meyer and other competitors, a more effective evangelist for the periodic system. Mendeleev took every opportunity to argue, at times heedlessly, that the characteristics of the elements repeat in an orderly and predictable way. He was both indefatigable and inflexible, at least until the tide of scientific opinion turned against him. In the late eighteen-fifties, scientists found that the elemental makeup of a given substance could be deduced from the light that it gave off when set ablaze; in 1868, a French astronomer, Jules Janssen, used the technique to discover helium (element No. 2) on the surface of the sun, during a total solar eclipse. At first, Mendeleev argued that helium could not exist; it had no place on the periodic table. But, around the turn of the twentieth century, after the other noble gases had been discovered and shown to share properties with helium, other scientists made a column just for them, and Mendeleev fell in line. (The column runs along the right, with helium poking out on top).
Từ vựng tham khảo:
Glassblower (Noun) /ˈɡlæsˌbloʊ.ər/: Thợ thổi thuỷ tinh.
Furnaces (Noun) /ˈfɜːr.nəs.ɪz/: Lò đốt.
Phosphorus (Noun) /ˈfɑːs.fər.əs/: Photpho.
Gunpowder (Noun) /ˈɡʌn.paʊ.dər/: Thuốc súng.
Sulfur-coated (Adjective) /ˈsʌl.fɚˌkoʊt.ɪd/: Phủ lớp lưu huỳnh.
Apothecary (Noun) /əˈpɑː.θəˌker.i/: Dược sĩ, thầy thuốc.
Antimony (Noun) /ˈæn.təˌmoʊ.ni/: Antimon.
Ichthyosauros (Noun) /ɪkˌθaɪ.əˈsɔːrəs/: Thằn lằn cá.
Periodic table (Noun) /ˌpɪr.iˈɑː.dɪk ˈteɪ.bəl/: Bảng tuần hoàn.
Atom (Noun) /ˈæt.əmz/: Nguyên tử.
Alchemy (Noun) /ˈæl.kə.mi/: Giả kim thuật.
Atomistic ideas (Noun) /ˌæt.əˈmɪs.tɪk ˈaɪ.di.əz/: Các ý tưởng nguyên tử học.
Elemental schemes (Noun) /ˌel.ɪˈmen.t̬əl skiːmz/: Các kế hoạch nguyên tố.
Proliferated (Verb) /prəˈlɪf.ə.reɪ.t̬ɪd/: Phát triển mạnh, tăng nhanh.
Fizz (Verb / Noun) /fɪz/: Tiếng sủi bọt, tiếng tạo bọt khí.
Elemental prophecies (Noun) /ˌel.ɪˈmen.t̬əl ˈprɑː.fə.siːz/: Tiên tri về nguyên tố.
Gallium (Noun) /ˈɡæl.i.əm/: Galium.
Germanium (Noun) /dʒɝˈmeɪ.ni.əm/: Geman.
Evangelist (Noun) /ɪˈvæn.dʒə.lɪst/: Sứ giả Tin lành, nhà tiên tri.
Solar eclipse (Noun) /ˈsoʊ.lɚ ˈɪˌklɪps/: Nhật thực.
Tham khảo: Tổng hợp từ vựng tiếng Anh chuyên ngành Y cơ bản thường gặp
Tổng kết
Trên đây, bài viết đã chia sẻ tên tiếng Anh của các nguyên tố hóa học một cách đầy đủ và chính xác nhất, kèm theo đó là đoạn trích trong bài viết thú vị về lịch sử của bảng tuần hoàn hóa học. Với nguồn tài liệu này, tác giả bài viết hy vọng người đọc có thể nắm chắc kiến thức và vận dụng linh hoạt, hiệu quả trong công việc của mình.
Tài liệu tham khảo:
Jahromi, Neima. “The Histories Hidden in the Periodic Table.” The New Yorker, 27 Dec. 2019, www.newyorker.com/science/elements/the-histories-hidden-in-the-periodic-table




















![Ảnh Anime Cute Phô Mai Que Đẹp [102+ Hình Siêu Cute]](/uploads/blog/2024/11/25/abe5e97926c8d9f39bb0d04c91c956cbbc32fbca-1732503618.jpg)

